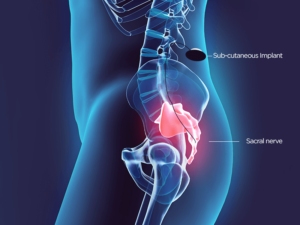
Sacral nerve stimulation (SNS) is a therapy that has been developed to help people who experience bladder and bowel problems such as fecal incontinence and constipation.
A device is surgically implanted and delivers electrical stimulation to the anal canal area, the lower part of the colon, and the bladder. If you have fecal (bowel) incontinence or chronic constipation, the use of this device may improve your ability to delay the urge to empty your bowel, decrease the number of accidents, and improve your quality of life.
FAQs
Sacral nerves are located in the pelvic area just above the tailbone. These nerves control the muscles and organs that contribute to overall bowel control, such as the anal sphincter and pelvic floor.
In some cases, SNS can effectively treat fecal incontinence. It may also be used to treat overactive bladder, including the frequent, sudden urge to go to the toilet. In addition, it can address symptoms of stubborn constipation and fecal incontinence.
Sacral nerve stimulation should be considered when dietary changes, medication, lifestyle changes, and other therapies are not successful. Individuals that are experiencing fecal incontinence and/or chronic constipation who have failed other treatments may be eligible for this treatment.
This treatment is performed in two stages. The first is the test stimulation phase to allow your physician to assess whether or not symptoms will be reduced from a temporary, external neurostimulator. You will be asked to document your symptoms during this trial period. If this therapy shows significant improvement for you during this time, then your physician will surgically implant a permanent stimulator for the next phase of treatment. This stimulator can last between 5-10 years before needing to be replaced.